Radioactivity surrounds us
Since the dawn of times, living beings have bathed in a flow of natural radiation. On a welcoming planet like Earth, this exposure is modest and has not prevented life from developing … Natural radioactivity is the principal source of radiation exposure for humans.
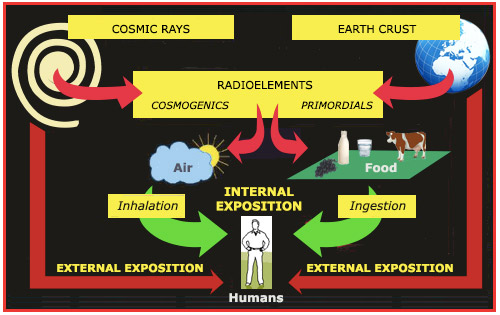
The paths followed by natural radioactivity
<radioactive atoms at the origin of natural radioactivity are present in the earth’s crust rocks since the formation of the Earth or formed permanently from cosmic radiation. In the first category, one finds uranium, thorium, their descendants including gaseous radon which passes through the atmosphere, and potassium-40. Cosmic radiation mainly produces carbon 14 which ends into vegetation. Cosmic rays and radiation from rocks are sources of external exposure for humans, while radioelements inhaled in the air or ingested through water and food generate internal exposure.
© IN2P3
In France, the exposure dose is 2.4 mSv (millisieverts) per person per year, as opposed to 1mSv from medical examinations. This is, of course, an average, and location and lifestyle play equal roles in determining the level of exposure. Where one travels, where one lives and even whether or not one uses air conditioning are all important factors.
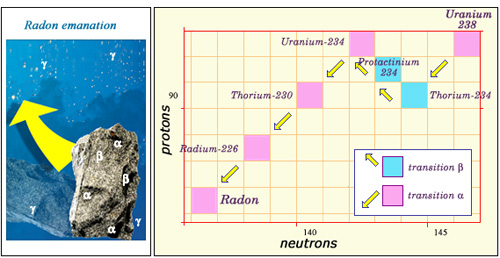
The natural radioactivity of rocks:
The majority of naturally-occurring radioactive elements are found in rocks. This nucleus graph shows the successive radioactive disintegrations that lead from uranium-238 to radon. The ‘descendants’ of uranium-238, present in trace amounts in minerals, emit alpha and beta rays which remain trapped inside the rocks. Gamma rays, on the other hand, are able to escape. The sixth descendant of uranium – radon, as shown above – is a gas. Amounts of it can therefore escape from the ground, thereby releasing radiation into the atmosphere.
© IN2P3
We are constantly being bombarded by particles of cosmic radiations : several hundred go through our bodies every second. Rocks like granite, which have become symbols of permanence and durability, contain light traces of radioactive uranium. Sitting on or walking near a block of granite exposes you to previously not encountered sources of radioactivity.
Even the food we eat or the air we breathe contains radioactive elements – either formed thanks to the intervention of cosmic rays, or as old as the solar system itself. There is absolutely no way to escape from it: even we are radioactive! Eight thousand atoms of potassium 40 or carbon 14 disintegrate in our bodies every second.
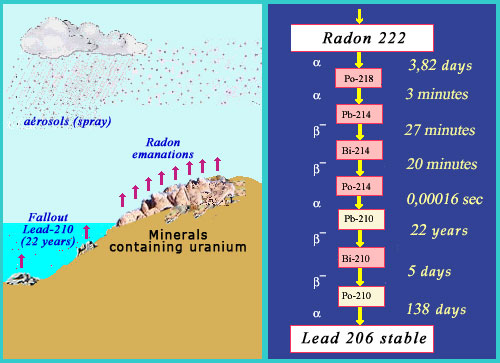
The role of radon
Continuation of the uranium decay chain. Radon, which as a gas can escape from its underground jail, decays comparatively quickly into lead-210: a radioactive substance which survives for decades. The chain finally ends with lead-206, a stable lead isotope. If the radon source is deep underground, then radon may not have the time to reach the surface; in which case, the radioactivity stays in the soil. If the radon does manage to break through, however, some of its decay products can be harmful to human lungs.
© IN2P3
The most important natural source of radioactivity is a rare gas known as radon. One of the products of uranium decay, radon is an ‘inert gas’ that can participate in no chemical reaction. This would seem to make it completely harmless, were it not for the fact that radon own radioactive decay produces gases poisonous to humans. The nature of the soil you live on, the construction tools used to build your house and the quality of its air conditioning are all crucial factors in determining radon exposure.
It is important to stress, at this point, that 2.4 mSv is a low dose. In certain parts of India, China and Brazil the natural exposure level rises to 10 or even 20 mSv a year. The fact that humans have survived these comparatively overexposed conditions indicates that all doses of the order of millisieverts are negligible and very likely harmless.
Articles on the subject « Natural Radioactivity »
Natural Origins
From vestiges of earth formation to cosmic rays Despite having evolved under constant exposure to[...]
Uranium and thorium origins
Radioactive substances older than the Earth The principal source of natural radiations on Earth i[...]
Cosmogenic Radioelements
Formation of radioactive atoms from cosmic rays The Earth is constantly being bombarded by ‘[...]
Natural Exposure
A chronic but benign exposure All exposure to radioactivity, whether natural or artificial, is me[...]
Ground Radioactivity
Telluric Exposure : Radiation which emanates from rocks The Earth crust contains a number of radi[...]
Human Internal Exposure
Our bodies are also… slightly radioactive All through our lives, we inhale and ingest radio[...]
Radioactivity in food
In our glasses and on our plates … The very acts of breathing and walking around make it im[...]